-

GTH 2026 Bonn
Visit us from
February 17th - 20th, 2026 at the GTH in Bonn,
booth nr. 27
-

Discounted Products
Special pricing for products which will expire in 6 months or less. For more details see our news article.
-

Reagents & Kits for coagulation diagnostics by Pentapharm:
Haemostasis kits (e.g. Pefakit® APC-R Factor V Leiden); Peptide substrates; Snake venom enzymes
-

Factor VIII antibodies ("ESH-series")
Now available ESH-4, ESH-5 and ESH-8 antibodies. Please send an e-mail (info@loxo.de) if you need a quote.
-
Haemostaseological diagnostics in connection with COVID-19
Of interest to you? Please send us an email to (info@loxo.de).
-

Products from Innovative Research for your studies
Looking for human and animal biological products for your studies? Send your request to: info@loxo.de
-
BD QuikHeel™ for blood drawing from newborns and infants
Of interest to you? Please send us an email to (info@loxo.de).
-
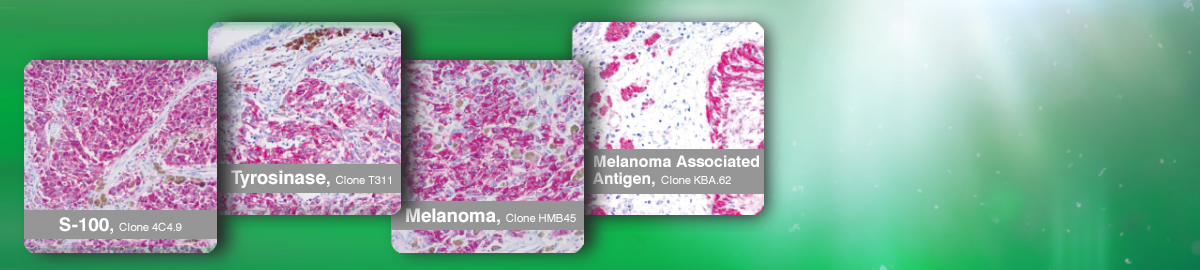
Melanoma-Diagnostics
Antibodies for Immunohistochemistry.
Of interest to you?
Please send us an email to (info@loxo.de).
Welcome to LOXO
LOXO GmbH distributes reagents and test systems for the medical diagnostic laboratory as well as for industrial and academic laboratories in medical and biological research.
The product range includes both research reagents (Research Use Only products; RUO) and in-vitro diagnostics (IVD; CE-marked).
30.07.2025
Human IgG3 ELISA Kit from Innovative Research
Looking to expand your immune profiling? Then consider the new Human IgG3 ELISA Kit from Innovative Research to measure this key immunoglobulin. Key ... Read more
29.07.2025
We moved!
We are happy to announce that we have successfully moved to new premises.
Our new postal address is
Dieselstr. 2, 69221 Dossenheim, Germany.
The ... Read more
03.07.2024
Discounted products update July 2024
Please find below a list of products which will expire in 6 months or less. Special pricing applies! Please inquire by e-mail: info@loxo.de or ... Read more
Product portfolio
LOXO supports diagnostic laboratories in the identification and IVDR-compatible implementation of new test procedures for innovative immunological and molecular biological test parameters, with a special focus/emphasis on hemostasis and fibrinolysis.
Main focus of the LOXO product portfolio:
- Blood coagulation and fibrinolysis: routine and special products
Reagents incl. in-vitro diagnostics (IVD) for determination of all factors and functions of the coagulation and fibrinolysis system, purified proteins/factors, antibodies, inhibitors, substrates (chromogenic and fluorogenic), controls and complete test kits.
- Platelets: function reagents and flow cytometric characterization
Ristocetin Cofactor assay kit, Platelets, Ristocetin, ADP, Arachidonic Acid, Collagen, Epinephrines, TRAP-6
In addition, an extensive range of monoclonal antibodies for flow cytometry of platelets with a broad spectrum of different fluorochrome labels; IVDR-compatible application protocols
-
Consumables for coagulation diagnostics and disposiable devices
Bleeding time (Surgicutt), Newborn capillary blood collection (QuikHeel, tenderfoot)
- Blood Group Test Sera
AB0 System, Rhesus System, Kell, Duffy, Bromelin, Bovine Albumine, Anti-Human Globulin, Bedside Test cards, and much more
-
Histology: From basic reagents (staining solutions) to antibodies and detection systems for immunohistochemistry (IHC)
In-vitro-diagnostics (IVD) and research reagents (RUO)
- Molecular biology: From basic reagents to complete detection methods
In-vitro-diagnostics (IVD) and research reagents (RUO)
- Extensive range of ELISA kits including IVDR consultation
We search, find and procure for you
In cooperation with our partner organisation ImmBioMed GmbH & Co. KG, we can offer you advice and also assistance in test verification and validation under the provisions of the current In Vitro Diagnostics Directive (IVDR), especially related to so-called "Lab-Developed Tests (LDT)".
You can order information material, offers or the desired products simply via our shopping cart request function
Or send us an e-mail to info@loxo.de
or simply give us a call at: +49 6221 868023
We are looking forward to your inquiry!
Your
LOXO team
Quality management
In 2001, LOXO GmbH was successfully certified. Since 2004, its quality management has been meeting the requirements of DIN EN ISO 13485.